Every day we carry around 10 times more microbial cells than our own. Yes, the vast majority of cells in the human body are not human, but are in fact bacteria that coexist peacefully and hitchhike in us. The makeup of our microbes may explain why people with certain lifestyles are at risk for developing obesity, asthma, and other common Western diseases.
Our microbial partners carry out a number of metabolic reactions that the human genome does not encode but are necessary for human health. Although we haven’t figured out how to isolate and grow most of the microscopic bugs that live in different parts of our bodies, Next Generation Sequencing (NGS) and metagenomics are allowing us to identify the microbial communities, their genes, and their key roles in health and disease. Perhaps most studied area is the influence of gut microbiota on a person’s risk of obesity.
Our microbiota is what makes us very different
According to JAX Professor Dr. George Weinstock we have 100 times more microbial genes than we have human genes and microbial genes are binding and changing things within us. As Weinstock describes it, “So you’re not just a human, you’re kind of a super organism because you’re a community of all these things that are with you your whole life”. He explains “All of these microbial genes make up what is called your second genome or metagenome – a big genome made up of a bunch of little genomes.” Dr. Weinstock, is also a leader of the Human Microbiome Project.
The Human Genome Project showed us in 2003 that 99.9 percent of our genomes are identical in every person. Ten years later, the Human Microbiome Project indicated for the first time that our main genetic differences are actually in our microbiomes. The mix of microbes we host is unique to each individual. Even couples that have been together for decades have their own distinct mix of microscopic bugs. It remains unknown how and why these microbial communities vary so extensively amongst individuals.
Who lives within us?
Alright, we have trillions of single cell organisms living in us, but who are they and where do they live? More than 70% of our microbes live in the colon. Other microbial communities colonize other anatomical regions such as the mouth, skin, hair, and genital areas. The vast majority of them are bacteria, although viruses and fungi are also part of our microbiome.
Although over 100 bacterial phyla have been described, two bacterial phyla account for more than 90% of bacterial cells: Firmicutes and Bacteroidetes. Smaller populations of Proteobacteria, Verrucomicrobia, Actinobacteria, Fusobacteria and Cyanobacteria are also present in different proportions around the human body. Exploring the microbiome of over 300 adult Europeans and North Americans with new tools may redefine concepts of what “healthy” or “at risk for disease” mean as well as shed some light on how Western life-styles are affecting us. It is becoming clear that both the microbiome composition and function impact human health.
The complex interactions between microbial communities and their hosts involve functions such as defense, metabolism and reproduction. The function of our microbiome can be altered by changes in microbial populations, dietary changes, or exposure to antibiotics. Promising research using animal models and human patients shows that manipulating bacterial communities can dramatically affect an individual’s health through modulating risk for obesity, colitis, asthma or allergies, or the risk for developing specific types of cancer.
Most of the work so far has been looking at gut flora and how it strongly influences the development and maintenance of our immune system, which acts as a barrier from pathogen invasion. The gut flora also makes many nutritional contributions, including breaking down indigestible food and absorption of nutrients to producing vitamins and amino acids. In fact, our microbiota generates most of the metabolites that are detected in plasma.
Dysbiosis: when our internal ecosystem goes wild
There is consensus on the fact that changes in the structure and composition of the commensal microbiota have an impact on human health. Dysbiosis is the condition that often happens when the normal microbiome population structure is disturbed—for example by external pressures such as disease states or the unintended collateral damage of antibiotics. In such states, the “good” bacteria no longer are able to control the “bad” ones. Such disruptions of the microbial ecosystem are linked with human diseases including autoimmune diseases, inflammatory disorders, and obesity.
Lifestyle seems to strongly influence in our microbiome. A popular working hypothesis is that chronic diseases affecting more than 50% of the adult population in Western countries may be strongly influenced by the combination of Western diets (based on red meat, animal fat, high sugar, and low fiber foods) repeated use of antibiotics, and a sedentary lifestyle.
Is obesity a state of gut?
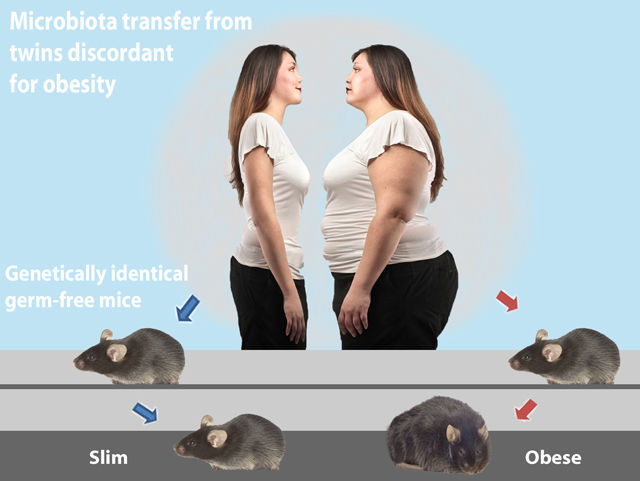
On one hand, our genetic makeup determines what kind of bacteria reside in our gut. On the other hand, the bacterial composition of our gut influences our body mass and adiposity. Interestingly, transferring the microbes from an obese person into mice raised with no microbes of their own results in fatter mice. (Figure 1). Experiments transplanting gut flora from slim mice dramatically changes the phenotype of genetically identical recipient mice (Figure 2).
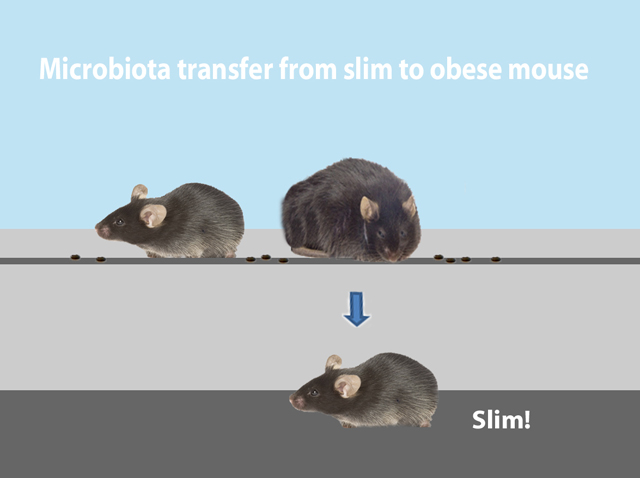
This experimental approach provides opportunities to figure out which bacterial taxa populate the gut communities of cage mates, how the change in the microbial constituency correlates with host phenotypes, and how diet affects the microbial niche. Additional research highlights the impact of repeated antibiotic use on our internal ecosystems. For example, administration of antibiotics before the age of 6 months was significantly associated with development of obesity later in life.
Although there are plenty of “microbiomaniacs” that oversell the significance of the microbiome and a probiotic market that is growing through the roof, there is no question that our microbes impact our health in many ways. One of the biggest challenges in the field right now is to discover if there is a causal link between microbiome variation and a variety of pathological states. Is dysbiosis a consequence of chronic inflammation or is it the main trigger that leads to it? Fundamental questions about these newly appreciated host-microbiome interactions are still open.
The discovery of our metagenome has changed our perspective of ourselves. As microbiome researcher Dr. Jeffrey Gordon put it, recent developments are “allowing us to see ourselves as intimately connected with the microbial world.” There is great promise that carefully controlled microbiome R&D efforts will bring prebiotic and probiotic therapies designed to improve health through mechanisms focused on host-microbiome interactions. In the meantime, we’ll be waiting—with 100 trillion of our very closest friends.