Researchers at The Jackson Laboratory and UConn Health are rigorously investigating why vaccines don’t work as well in some older adults.
An important aspect to aging is how the immune system changes over time. Such changes have consequences, and they contribute to the greater risk in the aging population for severe infections and other diseases, such as cancer. Age-related changes in the immune system also play a role in variable responses to vaccines and overall lower efficacy of vaccines compared to younger adults. Researchers at The Jackson Laboratory and UConn Health are rigorously investigating why vaccines don’t work as well in some older adults.
Protection against pneumococcal infections
Streptococcus pneumoniae is a dangerous bacterial pathogen that causes diseases such as pneumonia, meningitis and sepsis. Infants and older adults are at greatest risk for pneumococcal infections, and case-fatality rates increase with age for reasons that are still not well understood. Fortunately, several vaccines developed against the polysaccharides found on the surface of S. pneumoniae, including PPSV23 (Pneumovaxâ), are generally effective in older adults, though not as protective as in younger adults. Combining (conjugating) the polysaccharide with a protein, such as a nontoxic variant of a diphtheria toxin, can induce additional adaptive immune activation, resulting in better protection. The strategy was used to develop a new class of FDA-approved conjugated vaccines (e.g., PCV13, Prevnarâ). Despite these advances, responses to pneumococcal vaccines still decline with age. Moreover, it remains unclear which of these two vaccines is preferable in subpopulations of older adults.
To address these gaps in knowledge, a team led by JAX Associate Professor Duygu Ucar, Ph.D., UConn Health Professor and Director of UConn Center on Aging George Kuchel, M.D., C.M., and Jacques Banchereau, Ph.D. (Immunoledge, Montclair, NJ), recruited and vaccinated a cohort of 39 pneumococcal vaccine-naïve healthy adults, all aged 60 or above, to thoroughly compare pre- and post-vaccine immune characteristics. Their findings, presented in “Distinct baseline immune characteristics associated with responses to conjugated and unconjugated pneumococcal polysaccharide vaccines in older adults,” published in Nature Immunology, identify the biological traits underlying variable responses to the two different vaccines. Importantly, they also reveal distinct baseline (i.e., pre-vaccination) predictors that have the potential to affect vaccination strategies and lead to interventions that are more effective, by virtue of being more specific.
“Understanding who will respond strongly to which vaccine will provide us opportunities to stratify the population for improved vaccine efficacy at the population level, as well as understanding whether we can modulate the immune characteristics of individuals prior to vaccination to improve outcomes at the individual level,” says Ucar.
Efficacy indicators
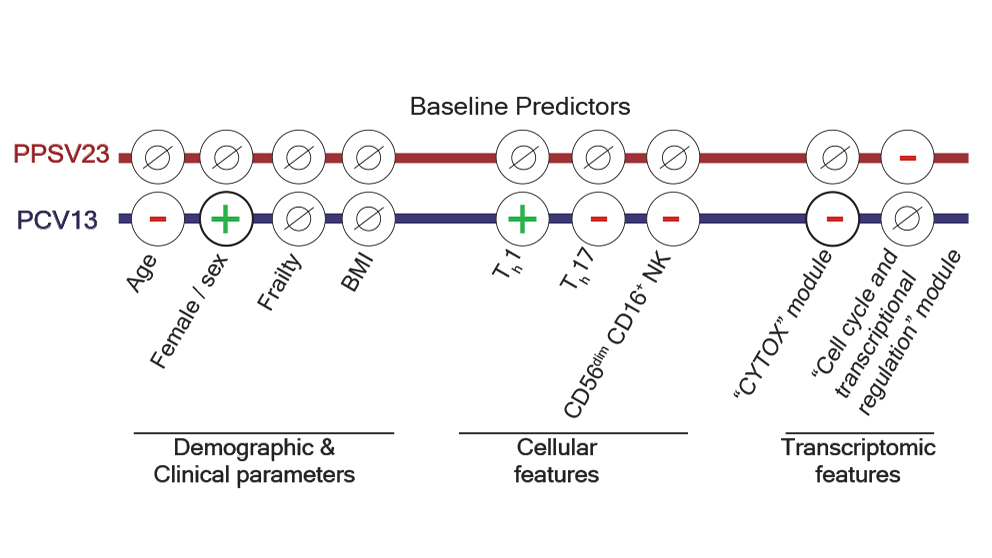
All participants received a single dose of PPSV23 or PCV13 between May and the early fall. Blood was drawn before vaccination, then 1, 10, 28 and 60 days afterward to provide longitudinal data. Following vaccination, the researchers developed measures to quantify vaccine responses and rank donors with respect to responsiveness within the cohort. While overall responses to both vaccines were comparable, there were clear differences in baseline immune phenotypes, separating the strong and weak responders.
The baseline abundance of two specific T cell types, Th1 and Th17, played an important role in PCV13 responses. Th1 cells produce molecular signals to activate early innate immune responses to pathogens, while Th17 cells also contribute to the defense response by producing a different group of inflammatory signaling molecules. For PCV13 vaccine responses, higher levels of Th1 cells showed a positive association and higher levels of Th17 cells a negative association. Thus, a pre-vaccination Th1/ Th17 ratio can be predictive of PCV13 response strength. Interestingly, women have a higher frequency of Th1 and lower frequency of Th1 7 cells compared to men and responded more strongly to the PCV13 vaccine.
From the pre-vaccination gene expression data, the researchers uncovered a gene module that included cytotoxic genes that was associated with reduced PCV13 responses, called the CYTOX signature. Single cell profiling linked this gene expression signature to mature CD16+ Natural Killer (NK) cells. The abundance of mature CD16+ NK cells in blood was associated with responses to PCV13, where weak responders had more CD16+ NK cells than did strong responders. The CYTOX signature was not associated with responses to the alternative PPSV23 vaccine, however—another, distinct gene set predicted responses to PPSV23.
“Our study offers a reminder that ‘one size fits all’ approaches do not work well for older patients,” says Kuchel. “Moreover, if our findings can be replicated in other populations, they may offer remarkable opportunities for implementing care models involving Precision Gerontology that are more effective for older adults by virtue of being more precise, ultimately matching individuals with those vaccines that work best for them.”
Implications for disease prevention
A surprising aspect of the study is that the baseline predictors for the two available classes of pneumococcal vaccines are quite distinct and independent from each other, despite both vaccines using the same bacterial polysaccharides to provoke the protective immune response. Importantly, however, the paper shows that responses to the two vaccines can be predicted in older adults based on specific pre-vaccination characteristics, and the findings imply that individuals can be readily stratified based on which vaccine is likely to work best for them. For example, older adults with low CYTOX/CD16+ NK cell levels will likely respond well to the PCV13 vaccine, while those with high CYTOX would more likely benefit from the PPSV23 vaccine. Overall, the results have important implications for more precise vaccination strategies for pneumococcal vaccines, and potentially for other vaccines as well, so that older adults can be better protected from infection and disease.