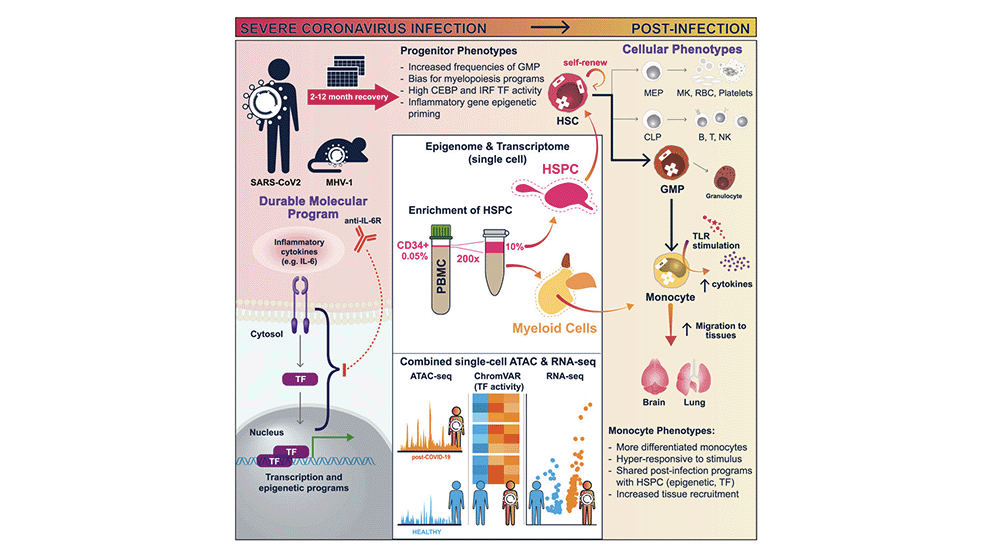
Severe COVID-19 triggers changes that affect gene expression in immune system stem cells, causing long-lasting alterations in the body’s immune response, according to a new study by Weill Cornell Medicine and Jackson Laboratory investigators. The finding could help explain symptoms of prolonged inflammation and long COVID in people who have had the disease.
The research team, led by Steven Josefowicz, Ph.D., an associate professor of pathology and laboratory medicine at Weill Cornell Medicine, and Duygu Ucar, Ph.D., an associate professor at The Jackson Laboratory for Genomic Medicine, published Epigenetic Memory of COVID-19 in Innate Immune Cells and Their Progenitors in Cell on August 18. For the study, the team developed a new technique to isolate and analyze rare stem cells found in human blood called CD34+ hematopoietic stem and progenitor cells.
Examining the epigenetic landscape
Using such cells from patients who had recovered from severe COVID-19, they examined changes to the way the DNA was packaged and condensed, collectively known as the epigenetic landscape. These epigenetic alterations determine the probability or level that a gene will be turned on in both the stem cells and their offspring, which are mature immune cells. The investigators found that the patients’ stem cells acquired more accessible DNA, thereby permitting gene activation, especially at genes that drive inflammation and genes that determine whether the cells develop into inflammatory cell types. The mature immune cells that derived from these stem cells were also sensitized to future encounters with pathogens.
“All immune cells and all blood cells come from hematopoietic stem cells,” says Josefowicz. “We found that these stem cells can pass their epigenetic ‘memories’ on to their progeny immune cells, changing those cells’ inflammatory programs. So, when they see another pathogen, they respond in a different way than they would if they came from progenitor cells that hadn’t seen inflammation to the same extent.”
It is well known that exposure to a virus, such as SARS-CoV-2, causes adaptive immune cells cells to respond to fight the diseases (for example, by producing antibodies), and eventually form a “memory” of past infections, enabling them to recognize and fight future infections by the same virus faster and more effectively. But scientists are just beginning to understand immune memory in innate immune cells, a different group of cells that first respond to an infection, before antibody-producing cells are activated. The process is thought to occur through epigenetic changes to hematopoietic stem cells.
A novel approach for researching hematopoietic stem cells
Research on hematopoietic stem cells, which are most abundant in bone marrow, has been limited by the costly and invasive techniques required to profile these cells. However, using their novel approach for isolating, enriching and studying hematopoietic stem cells circulating in the blood, the team demonstrated that these hematopoietic stem and progenitor cells—0.05 percent of all circulating peripheral blood mononuclear cells—capture the full cellular diversity of their bone marrow counterparts. This revelation opened the doors to study, at single cell resolution, how stem cells are affected upon infection and vaccination with a simple blood draw.
The team, including first author Jin-Gyu Cheong, Ph.D., a postdoctoral associate in the Josefowicz lab and recent graduate from the Weill Cornell Medicine Immunology and Microbial Pathogenesis graduate program, applied this technique to thoroughly characterize how severe SARS-CoV-2 infections affect the epigenetic state of human hematopoietic stem cells and implications of these changes for the future immune responses. They observed that the epigenetic changes persisted over time; although the patients had recovered months ago, their stem cells still carried the epigenetic signatures of the disease.
In addition to looking at stem cells, the team also looked at monocytes, a type of white blood cell involved in the innate immune response that are newly minted every few days from stem cells. They found that these cells had different epigenetic programming up to one year after a severe COVID-19. Alterations included changes that made these immune cells hyper-responsive to stimulation.
Focusing on stem cells
Next, the researchers took a closer look at the stem cells themselves. They found that after COVID-19, these cells had changes to their transcription factor programming that made them more likely to create myeloid-type blood cells, the first responders to infection.
“It is fascinating that COVID-19 can induce such robust epigenetic changes in stem and progenitor cells as well as in their progeny mature innate immune cells, which can last for months and can reshape the innate immune responses to future immune threats,” says Ucar. “Understanding epigenetic memory associated with infections can be a way for us to understand how certain diseases like COVID-19 have such long-lasting health consequences. Furthermore, it provides us an opportunity to assess immune status at the epigenetic level upon infections and even upon vaccination.”
The researchers also looked at variables that could explain why some patients had more epigenetic changes than others. They found that COVID-19 patients who received a treatment to block IL6, a well-known inflammatory molecule, during acute disease, were less likely to have significant immune memory changes a year later. How IL6 and other factors present during an infection can program lasting changes in stem cells is an ongoing focus for the researchers.
Game on for blood stem cell research
Next, the researchers are interested in using this new platform to study hematopoietic stem cell sub-types, as they are much easier to access in the blood than from bone marrow. The work will help reveal how they are impacted by a variety of diseases, as well as how they might be affected by therapies.
“Studying how hematopoietic stem cells respond in the context of human disease has been a bit of a black box because it’s so hard to access these cells in bone marrow,” says Josefowicz. “With this workflow to study plasticity of blood stem cells from a simple blood draw, it’s now game on.”